Li Valence Electrons
The Lewis Structure (electron dot diagram) of each ion is used to construct the Examples Lithium fluoride, LiF Lithium atom loses one electron to form the. Lewis symbol for fluoride ion has 8 dots and a -1 charge.
- 3 Li - Lithium
- Li Element Valence Electrons
- Valence Electrons Calculator
- Level Of Valence Electrons
- How Many Electrons, Protons, And Neutrons Does Li^+ Have ...
- What Is Lithium Valence Electrons
There are two ways to find the number of valence electrons in Lithium (H). The first is to use the Periodic Table to figure out how many electrons Lithium ha. .Valence electrons are electrons in the outer energy level of an atom.
. There's not enough electrons available in the structure for each atom to have an octet by themselves; . Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).
How do you count the electrons in a Lewis dot structure? BeF 2 forms, with one beryllium central to the two fluoride ions. each fluorine has seven electrons to. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).2.
The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1.
Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F Lithium fluoride compound can be represented as Li + OR 1. And thus the neutral atom has 7 valence schematron.org course the elemental form is bimolecular.
Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).. Which do you think would be bigger; fluorine atom or fluoride ion? Lewis Structure (electron dot diagram) for hydrogen fluoride OR.
The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond). The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound.
Examples. Lithium fluoride, LiF. Lithium atom loses one electron to form the cation Li+.
Fluorine atom gains one electron to form the anion F- Lithium fluoride compound can be represented as. Li+ OR. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
The number of dots equals the number of valence electrons in the atom.What is the Lewis electron dot structure for a fluoride ionWhat is the Lewis electron dot structure for a fluoride ion
Element Lithium - Li
Comprehensive data on the chemical element Lithium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Lithium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Lithium Menu
- Lithium Page One
- Lithium Page Two
- Lithium Page Three
Overview of Lithium
- Atomic Number: 3
- Group: 1
- Period: 2
- Series: Alkali Metals
Lithium's Name in Other Languages
- Latin: Lithium
- Czech: Lithium
- Croatian: Litij
- French: Lithium
- German: Lithium - s
- Italian: Litio
- Norwegian: Litium
- Portuguese: Litio
- Russian: Литий
- Spanish: Lítio
- Swedish: Litium
3 Li - Lithium
Atomic Structure of Lithium
- Atomic Radius: 2.05Å
- Atomic Volume: 13.1cm3/mol
- Covalent Radius: 1.23Å
- Cross Section (Thermal Neutron Capture) σa/barns: 70.5
- Crystal Structure: Cubic body centered
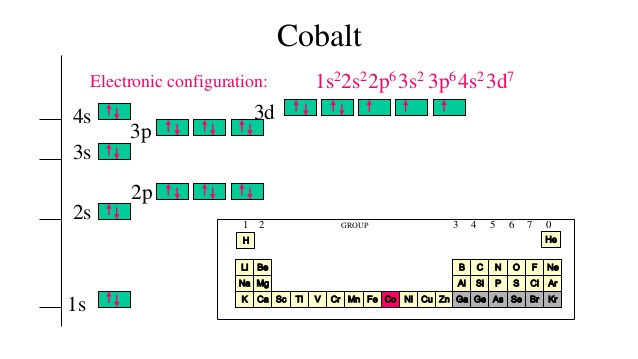
- Electron Configuration:
- 1s2 2s1
- Electrons per Energy Level: 2,1
- Shell Model
- Shell Model
- Ionic Radius: 0.76Å
- Filling Orbital: 2s1
- Number of Electrons (with no charge): 3
- Number of Neutrons (most common/stable nuclide): 4
- Number of Protons: 3
- Oxidation States: 1
- Valence Electrons: 2s1
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Lithium
- Electrochemical Equivalent: 0.259g/amp-hr
- Electron Work Function: 2.9eV
- Electronegativity: 0.98 (Pauling); 0.97 (Allrod Rochow)
- Heat of Fusion: 3kJ/mol
- Incompatibilities:
- water, acids, oxidizing agents
- Ionization Potential
- First: 5.392
- Second: 76.638
- Third: 122.451
- Valence Electron Potential (-eV): 19
Physical Properties of Lithium
- Atomic Mass Average: 6.941
- Boiling Point: 1615.15K 1342°C 2448°F
- Coefficient of lineal thermal expansion/K-1: 56E-6
- Conductivity
- Electrical: 0.108 106/cm Ω
Thermal: 0.847 W/cmK
- Electrical: 0.108 106/cm Ω
- Density: 0.534g/cc @ 300K
- Description:
- Soft silvery-white metal. Lightest of metals.
- Elastic Modulus:
- Bulk: 11/GPa
- Rigidity: 4.24/GPa
- Youngs: 4.91/GPa
- Enthalpy of Atomization: 160.7 kJ/mole @ 25°C
- Enthalpy of Fusion: 3 kJ/mole
- Enthalpy of Vaporization: 134.7 kJ/mole
- Flammablity Class: Flammable solid
- Freezing Point:see melting point
- Hardness Scale
- Mohs: 0.6
- Heat of Vaporization: 145.92kJ/mol
- Melting Point: 453.85K 180.7°C 357.3°F
- Molar Volume: 13 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 3.6J/gK
- Vapor Pressure = 1.63E-08Pa@180.7°C
Regulatory / Health
- CAS Number
- 7439-93-2
- UN/NA ID and ERG Guide Number
- UN1415 / 138
- RTECS: OJ5540000
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 0.004
- Bone/p.p.m: 1.3
- Liver/p.p.m: 0.025
- Muscle/p.p.m: 0.023
- Daily Dietary Intake: 0.1-2 mg
- Total Mass In Avg. 70kg human: 7 mg
Who / Where / When / How
- Discoverer: Johann A. Arfvedson
- Discovery Location: Stockholm Sweden
- Discovery Year: 1817
- Name Origin:
- Greek: lithos (stone)
- Abundance of Lithium:
- Earth's Crust/p.p.m.: 20
- Seawater/p.p.m.: 0.17
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 10
- Sources of Lithium:
- Spodumene, ambylgonite, lepidolite and desert lake brines. Also obtained by passing electric charge through melted lithium chloride. Around 39,000 tons of lithium is produced each year. The primary source of lithium is the USA.
- Uses of Lithium:
- Used in batteries, ceramics, glass, lubricants, alloy hardeners, pharmaceuticals, hydrogenating agents, heat transfer liquids, rocket propellants, vitamin A synthesis, nuclear reactor coolant, underwater buoyancy devices and the production of tritium. Deoxidizer in copper and copper alloys.
- Additional Notes:
- Lithium was first isolated in 1821 by W.T Brande. Near its melting point, lithium ignites in air. Lithium posses a dangerous fire and explosion risk when exposed to water, acids or oxidizing agents. It reacts exothermally with nitrogen in moist air at high temperatures. In solution lithium is toxic and targets the central nervous system.
Lithium Menu
- Lithium Page One
- Lithium Page Two
- Lithium Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
Li Element Valence Electrons
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Valence Electrons Calculator
Citing this page
Level Of Valence Electrons
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Lithium - Li. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/Li.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Li.html'>echo Periodic Table of Elements: Lithium - Li (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Lithium - Li is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
How Many Electrons, Protons, And Neutrons Does Li^+ Have ...
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
What Is Lithium Valence Electrons
PLEASE, if you like an article we published simply link to it on our website do not republish it.
